Author:
Stanley R. Kay, PhD Lewis A. Opler, MD Abraham Fiszbein, MD
Age Range:
18+
Scoring Option:
Hand scored
Publication date:
2006
Qualification level:
B
Completion time:
30-40 minutes per form
Forms:
Professional – Number of items: 33, Observer (IQ-PANSS) – Number of items: 98, Interview (SCI-PANSS) – Number of items: 179
Norms:
Normative data was gathered from a sample of 240 medicated schizophrenics. The major scales were all found to exhibit the characteristic Gaussian (bell-shaped) distribution curves, without significant skewness or kurtosis, which was taken to indicate that the scores are normally distributed in the comparison sample. Complete normative data are provided in the PANSS Manual.
The Positive and Negative Syndrome Scale (PANSS™) is based on findings that schizophrenia comprises at least two distinct syndromes: the positive syndrome, consisting of productive symptoms; and the negative syndrome, consisting of deficit features. It is useful when developing treatment plans because you can focus on the type of symptoms the patient is experiencing. The PANSS is also helpful when studying the effects of medication (e.g., in clinical drug trials) because it allows you to determine which type of symptoms are being affected.
Key Areas Measured
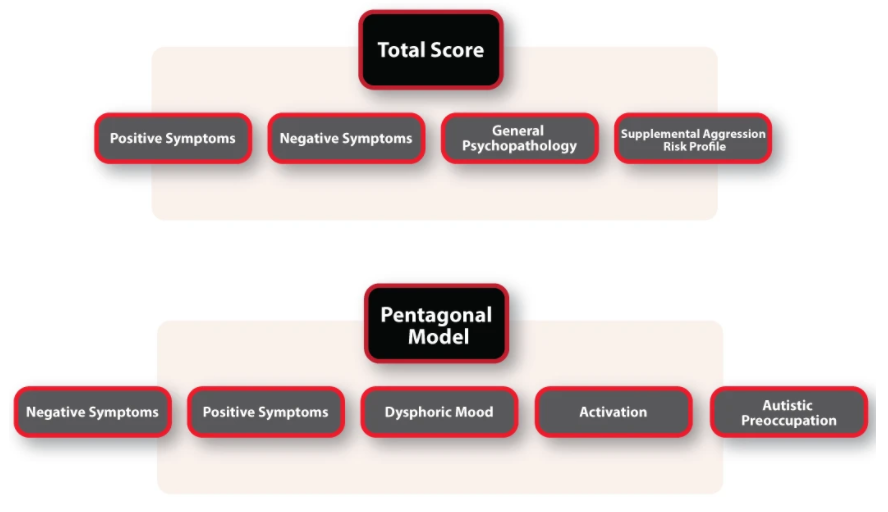
- Positive Scale
- Negative Scale
- General Psychopathology Scale
- Supplemental Aggression Risk Profile
Components
Structured Clinical Interview for PANSS (SCI-PANSS)
To optimize the instrument’s objectivity, the SCI-PANSS was developed to secure reliable information. The SCI-PANSS can be used to gather information objectively prior to completing the PANSS Rating and Profile Form.
Informant Questionnaire (IQ-PANSS)
The IQ-PANSS evaluates phenomenological presentation. Through item definitions, statements, and questions, the questionnaire ensures that all information related to behavioral presence, frequency, and responsiveness is obtained during interviews with family members, healthcare providers, or other informants. The IQ-PANSS information is then used to assist in completing the PANSS Rating and Profile Form.
How to Use
The PANSS is a hand scored instrument. It can be scored using the standard Dimensional scoring or the Pentagonal scoring method. The Pentagonal method uses 25 PANSS items organized into five scales: Negative, Positive, Dysphoric Mood, Activation, and Autistic Preoccupation.
Reliability and Validity
Thirty years of research support the use of the PANSS as a psychometrically sound measure of the presence and severity of symptoms of schizophrenia. The scales have demonstrated excellent internal consistency (coefficient alpha and split-half reliability) and consistency over time (test-retest reliability) while still being sensitive to change. The PANSS Positive and Negative symptom ratings are highly correlated with the Anderson method for evaluation, and the General Psychopathology scale has demonstrated significant associations with the NIMH Clinical Global Impression Scale.
Psychopharmacological research supports the PANSS’ construct, discriminative, convergent, and predictive validity. In addition, the American Food and Drug Administration (FDA) is supportive of the use of the PANSS as an outcome measure in clinical trials assessing drug efficacy, given the volume of studies that support its sensitivity to medication and other treatment effects when used longitudinally.






